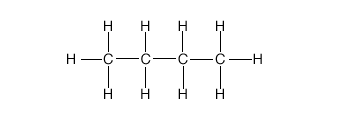
1. Two hydrocarbons are called isomers of one another
if they have the same number of hydrogen and carbon atoms but their structural
formulas (graphs) are not equivalent. The graph for butane is shown
below.

(a) How many isomers are there of butane?
Draw their graphs.
(b) How many isomers of are there of the
hydrocarbon pentane, C5 H12?
Pentane has five carbon atoms and 12 hydrogen atoms per molecule.
Draw the graphs of all the isomers.
2. Determine if there can exist hydrocarbons (possibly with double bonds) with the numbers of hydrogen and carbon atoms listed below.
(a) 7 carbon atoms, 13 hydrogen atoms.
(b) 5 carbon atoms, 6 hydrogen atoms.
In each case, either explain why no such hydrocarbon exists or, if one does exist, draw its graph.
3. If a hydrocarbon has four double bonds and six carbon atoms, what is the largest number of hydrogen atoms it can have? Why? Draw the graph of one such hydrocarbon.